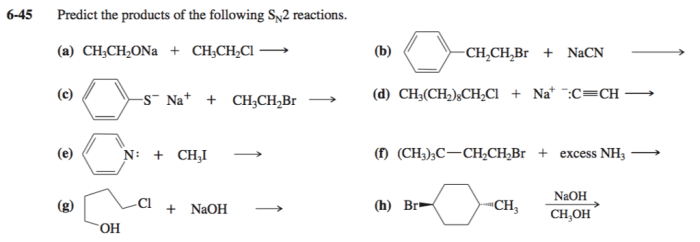
Predict the products of the following SN2 reactions: SN2 reactions are a fundamental type of organic reaction in which a nucleophile attacks an electrophile, resulting in the substitution of the electrophile’s leaving group with the nucleophile. Understanding the factors that influence SN2 reactions is crucial for predicting their products and applying them in organic synthesis.
This guide provides a comprehensive overview of SN2 reactions, including their mechanism, factors affecting their rate, and a step-by-step approach to predict their products. Additionally, it explores the stereochemistry of SN2 reactions and their applications in organic synthesis.
Introduction
SN2 reactions are nucleophilic substitution reactions in which a nucleophile attacks an electrophile, leading to the substitution of a leaving group. The mechanism of SN2 reactions involves a concerted, one-step process in which the nucleophile attacks the electrophile simultaneously as the leaving group departs.
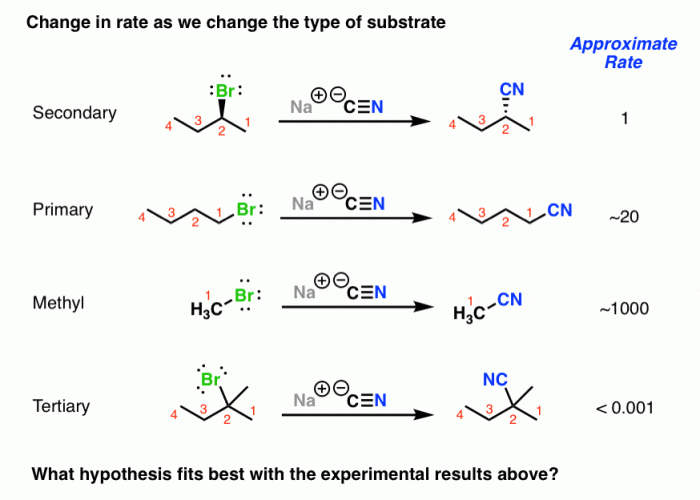
The rate of SN2 reactions is affected by several factors, including the nature of the nucleophile, electrophile, and leaving group, as well as the solvent and temperature.
Predicting Products of SN2 Reactions
To predict the products of SN2 reactions, the following steps can be followed:
- Identify the nucleophile, electrophile, and leaving group.
- The nucleophile attacks the electrophile at the carbon atom bearing the leaving group.
- The leaving group departs, and the nucleophile forms a new bond with the carbon atom.
- The configuration of the product is inverted compared to the starting material.
Examples of SN2 Reactions
| Starting Material | Nucleophile | Electrophile | Leaving Group | Product |
|---|---|---|---|---|
| CH3CH2Br | OH– | CH3CH2+ | Br– | CH3CH2OH |
| (CH3)3CBr | CN– | (CH3)3C+ | Br– | (CH3)3CN |
| CH3I | NH3 | CH3+ | I– | CH3NH2 |
Applications of SN2 Reactions
SN2 reactions are widely used in organic synthesis for the preparation of various compounds, including alcohols, ethers, and amines. These reactions are often used in the pharmaceutical industry to synthesize drugs and other biologically active compounds.
The advantages of using SN2 reactions include their high regioselectivity and stereoselectivity. However, SN2 reactions can be sensitive to steric hindrance and solvent effects, which can affect their yield and selectivity.
FAQ Guide: Predict The Products Of The Following Sn2 Reactions
What is the key difference between SN1 and SN2 reactions?
SN1 reactions proceed through a carbocation intermediate, while SN2 reactions occur in a single step.
How does the solvent affect the rate of SN2 reactions?
Polar aprotic solvents increase the rate of SN2 reactions by stabilizing the transition state.
What is the stereochemistry of SN2 reactions?
SN2 reactions typically proceed with inversion of configuration at the reaction center.